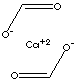CALCIUM FORMATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
544-17-2 |
|
| EINECS NO. | 208-863-7 | |
| FORMULA | (HCOO)2Ca | |
| MOL WT. | 130.12 | |
|
H.S. CODE |
2915.12.0000 | |
|
TOXICITY |
Oral, rat LD50: 2560 mg/kg |
|
| SYNONYMS | Formic acid calcium salt; Calcium diformate; Calcoform; | |
| Calciumdiformiat (German); Diformiato de calcio (Spanish); Diformiate de calcium (French); Mravencan vapenaty (Czech); | ||
| SMILES | C(=O)[O-].C(=O)[O-].[Ca+2] | |
|
CLASSIFICATION |
Formate |
|
|
EXTRA NOTES |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
White or light yellow powder | |
| MELTING POINT | > 300 C | |
| BOILING POINT |
| |
| SPECIFIC GRAVITY |
2.02 | |
| SOLUBILITY IN WATER |
Soluble | |
| SOLVENT SOLUBILITY | Insoluble in alcohol | |
| pH | 6.0-8.0 (25 C, 1 M in H2O) | |
| VAPOR DENSITY |
| |
| log P | -2.47 (Octanol-water) | |
| OH RATE CONSTANT | 0.0 (cm3/molecule-sec at 25 C Atmospheric) | |
| NFPA RATINGS | Health: 1; Flammability: 0; Reactivity: 0 | |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Drug Information Portal (U.S. National Library of Medicine) - CALCIUM FORMATE http://www.fhwa.dot.gov/ Local: DESCRIPTION OF FORMIC ACID: Formic acid, also called methanoic acid), is the simplest and has the lowest mole weight of the carboxylic acids, in which a single hydrogen atom is attached to the carboxyl group (HCOOH). If a methyl group is attached to the carboxyl group, the compound is acetic acid. It occurs naturally in the body of ants and in the stingers of bees. Functionally, it is not only an acid but also an aldehyde; it reacts with alcohols to form esters as an acid and it is easily oxidized which imparts some of the character of an aldehyde. Pure formic acid is a colorless, toxic, corrosive and fuming liquid, freezing at 8.4 C and boiling at 100.7 C. It is soluble in water, ether, and alcohol. It irritates the mucous membranes and blisters the skin. It is prepared commercially from sodium formate with the reaction of condensed sulfuric acid. Formic acid is used as a chemical intermediate and solvent, in processing textiles, leathers, electroplating, in coagulating latex rubber, and as a disinfectant. |
||
| SALES SPECIFICATION | ||
|
TECH GRADE |
||
|
APPEARANCE |
White or light yellow free flowing powder |
|
|
ASSAY |
98.0% min |
|
|
MOISTURE |
0.5% max |
|
|
FEED GRADE |
||
|
APPEARANCE |
white White or light yellow free flowing powder | |
|
ASSAY |
98.0% min |
|
|
CALCIUM |
30% min |
|
|
MOISTURE |
0.5% max |
|
|
HEAVY METAL |
20ppm max |
|
|
As |
3ppm max |
|
| TRANSPORTATION | ||
| PACKING | 25kgs, 500kgs , 1mt in Bag | |
| HAZARD CLASS | not regulated | |
| UN NO. | ||
| SAFETY INFORMATION | ||
|
GHS |
|
|
| SIGNAL WORD | Danger | |
|
PICTOGRAMS |
|
|
|
HAZARD STATEMENTS |
H318 Causes serious eye damage |
|
|
PRECAUTIONARY STATEMENTS |
P310 Immediately call a POISON CENTER or doctor/physician |
|
| EC DIRECTIVES |
|
|
| HAZARD CODES |
|
|
|
RISK PHRASES |
|
|
|
SAFETY PHRASES |
41 Risk of serious damage to eyes |
|
| PRICE INFORMATION | ||
|
|
||